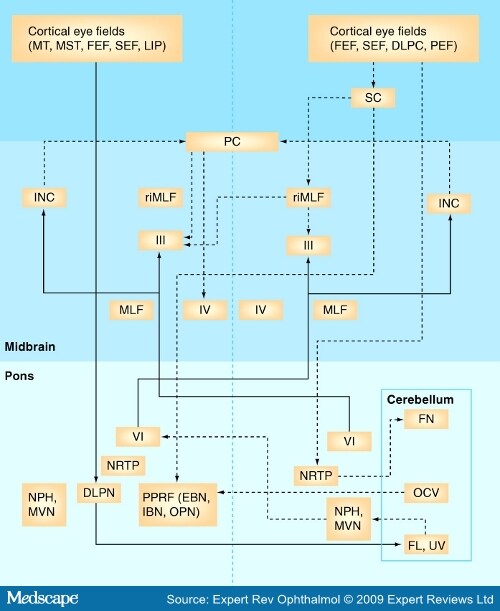
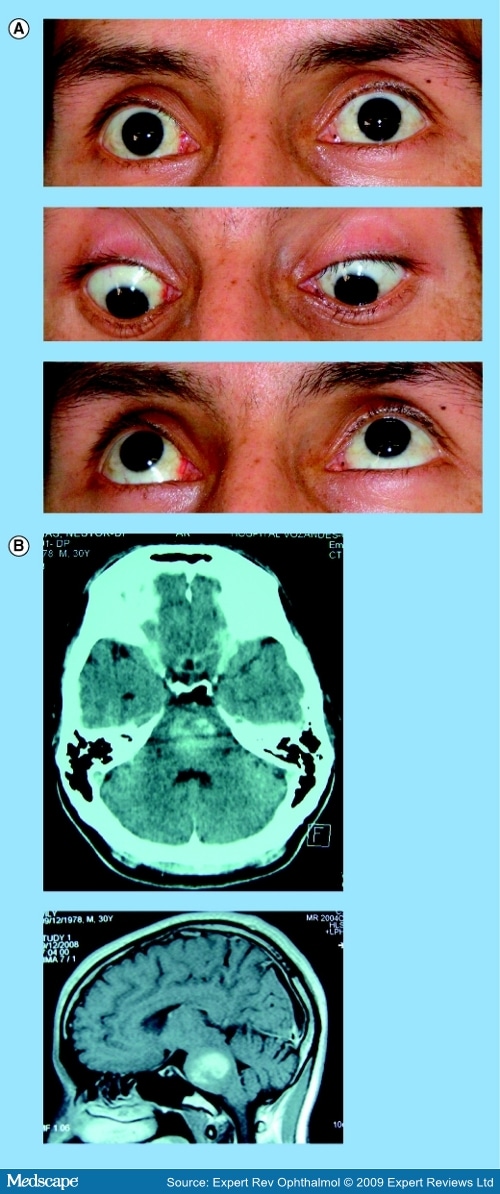
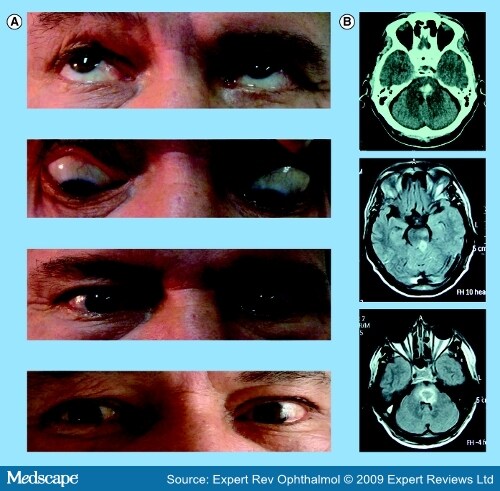
Oculomotor Disorders in Vertebrobasilar StrokeCME Released: 06/04/2009; Valid for credit through 06/04/2010 AbstractThe stunning and intricate interaction among the different neural circuits for all classes of functional eye movements requires the participation of the entire brain – from the cortex to the brainstem. The frequency of vertebrobasilar stroke has increased over the last two decades and emergency department physicians and specialists caring for stroke patients should, therefore, have the appropriate clinical skills for the timely recognition of posterior circulation strokes. Moreover, organization of the brainstem relative to vascular supply is key to a number of well-known syndromes whose clinical hallmarks may be oculomotor disorders. Diagnosis of brainstem stroke can often be challenging, particularly in the acute stage when CT scans and conventional MRI are normal or show only subtle changes. Consequently, stroke diagnosis continues to be based on clinical findings, where careful bedside clinical examination of eye movements can usually reveal a broad gamut of oculomotor abnormalities that are helpful in accurately determining the lesion site and, in some cases, may suggest the underlying etiology. This article summarizes the neural substrate of eye movements, the brainstem vascular supply and the different patterns of eye movement disorders due to stroke in the posterior circulation. IntroductionDuring the final decades of the 19th Century and the first half of the 20th Century, clinicians furnished a detailed description of the arterial anatomy of several brainstem structures, along with their pathological and clinical findings concerning ischemic or hemorrhagic brainstem stroke. Knowledge of the neural substrate participating in control of ocular movements partly arose from landmark descriptions of brainstem syndromes and was later complemented by the results of experimental animal model studies and, more recently, by modern neuroimaging techniques. Several areas of the brain are engaged in planning and executing ocular motor behavior. Specific cortical areas contribute to the initiation and control of voluntary eye movements. The brainstem and the cerebellum, which receive their vascular supply from vertebrobasilar system arteries, house a large number of critical structures or pathways involved in the generation, control or regulation of all the functional classes of eye movements. Infarcts predominate in posterior circulation stroke, as they do in the carotid circulation. In hospital-based stroke registries, 12–40% of infarcts involved tissue supplied by the vertebrobasilar circulation.[1-5] Over the last 25 years, there has been an increase in vertebrobasilar stroke, mainly ischemic, in both a community- and hospital-based stroke registry.[6,7] The middle territory (i.e., the pons and anterior inferior cerebellum) and the uppermost basilar artery territory are the sites most often involved in posterior circulation ischemia.[8] Eye movement disorders are commonly observed in patients with vertebrobasilar stroke, sometimes as isolated clinical findings, although they are more often associated with other signs of brainstem dysfunction. Eye Movement Disorders Occurring in Midbrain StrokeAnatomical Basis of Eye Motor Control at the MesencephalonThe neural substrate subserving vertical- and horizontal-gaze subsystems is organized differently. A detailed description of all complex pathways underlying generation and control of the functional classes of human eye movements is beyond the scope of this review. The mesodiencephalic junction is the critical site of prenuclear (supranuclear) vertical and torsional eye motion control.[9-12] At this level, the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF – the vertical-gaze center), the interstitial nucleus of Cajal (INC), the posterior commissure (PC) and the nucleus of Darkschewitsch – all clustered around the Sylvian aqueduct – are the anatomical structures mediating vertical-gaze inputs to the oculomotor nuclei.[13-17] The riMLF is located rostrally to the oculomotor nucleus, dorsomedially to the anterior pole of the red nucleus and ventrally to the periaqueductal gray matter.[15] It houses essential neurons for the generation of fast eye movements in vertical directions and torsional saccades.[15,18] At this level, the neurons for upward saccades are placed more laterally than those for downgaze.[10,11,15-17] The riMLF burst neurons fire at high frequencies, beginning just before the saccadic movement; otherwise, they are silent.[14] Moreover, the omnipause neurons located in the nucleus raphe interpositus cease firing during vertical and torsional saccades.[12] Inputs to each riMLF come from the superior colliculus, the nucleus of the PC, the contralateral riMLF, the fastigial nucleus of the cerebellum and the cerebral hemispheres.[12,13,18,19] From each riMLF, impulses traveling ventrally to the Sylvian aqueduct pass to the subnuclei of the eye muscles controlling upward and downward movements in both eyes, whereas impulses are uncrossed for torsional saccades.[20-22] Moreover, motoneurons for eye elevator muscles receive bilateral signals from the riMLF crossing through the oculomotor nuclear complex, while afferent signals for depressor muscles are only ipsilateral.[12,21] The INC (lying just below the riMLF) is important for integration of premotor signals (eccentric gaze holding or neural integrator) of the saccadic and torsional movements in vertical plane and head posture.[23-25] The neural integrator is defined as a neural circuit required for gaze maintenance, where the velocity-encoded motor command must be integrated to produce an appropriate eye position command or the gaze-holding mechanism.[12] The INC projects by way of the PC to motoneurons of the contralateral nuclei of the third and fourth cranial nerves and the contralateral INC.[12,26,27] Inputs to the INC come from the riMLF and caudal brainstem structures (i.e., the pons, medulla and the anterior semicircular canal) mainly by way of the MLF, the INC participates in vertical smooth-pursuit and vertical vestibular eye movements.[28] Finally, the PC contains fibers crossing from the INC to the contralateral structures involved in vertical gaze, while nuclei of the PC coordinate lid and eye movements occurring in saccade and pursuit movements in the vertical plane (Figure 1).[29,30] [ CLOSE WINDOW ] Figure 1.Main cortical and brainstem structures involved in generation and control of saccadic and smooth-pursuit movements in horizontal and vertical axes. Dotted lines indicate saccadic pathways. Solid lines indicate smooth-pursuit pathways. DLPC = Dorsolateral pontine cortex; DLPN = Dorsolateral pontine nucleus; EBN = Excitatory premotor burst neuron; FEF = Frontal eye field; FL = Flocculus; FN = Fastigial nucleus; IBN = Inhibitory premotor burst neuron; INC = Interstitial nucleus of Cajal; LIP = Lateral intraparietal area; MLF = Medial longitudinal fasciculus; MST = Medial superior temporal; MT = Middle temporal; MVN = Medial vestibular nucleus; NPH = Nucleus prepositus hypoglossi; NRTP = Nucleus reticularis tegmenti pontis; OCV = Oculomotor cerebellar vermis; OPN = Omnipause neuron; PC = Posterior commissure; PEF = Parietal eye field; PPRF =Paramedian pontine reticular formation; riMLF = Rostral interstitial nucleus of the medial longitudinal fasciculus; SC = Superior colliculus; SEF = Supplementary eye field; UV = Uvula; III = Oculomotor nucleus; IV = Trochlear nucleus; VI = Abducens nucleus. Midbrain Vascular SupplyAlthough the midbrain is a small anatomic structure, the blood supply is quite complex. An overlap between arterial territories of the basilar artery (BA), posterior cerebral artery (PCA) and superior cerebellar artery (SCA) can occur, and the degree of arterial contribution will depend on the midbrain level. The BA gives off direct perforators supplying the paramedian region (anteromedial territory), that is, short circumferential branches of the SCA that loop around the mesencephalon and feed the caudal two-thirds of the latero-dorsal region.[31,32] At the upper half of the midbrain, the proximal segment of the PCA branches into the thalamo–subthalamic and mesencephalic arteries. The former irrigate the riMLF, among other subthalamic structures.[33] Peduncular arteries, directed to the lateral upper midbrain stem, from the postcommunicating-PCA or P2 segment.[34] Midbrain StrokeMidbrain ischemia accounts for anywhere from 3 to 9.5% of all infarcts in the posterior circulation and often coexists with larger infarcts involving other brainstem arterial territories, mostly the neighboring pons and the thalamus.[31,32,35,36] Less often, midbrain ischemia may be exclusively restricted to the midbrain.[31,32,36] Up to 70% of patients with mesencephalic infarcts, commonly covering the middle and upper thirds, may exhibit a broad variety of neuro-ophthalmologic signs, sometimes in a complex pattern, which are usually observed with additional clinical features reflecting midbrain or brainstem involvement, but can also (less commonly) be isolated clinical findings.[31,32,35,36] Spontaneous hemorrhages rarely occur in the midbrain.[37-41] Supranuclear Eye Movement Abnormalities in Midbrain StrokeSupranuclear (prenuclear) vertical-gaze palsy, occurring in midbrain stroke, may be conjugate or disconjugate. Conjugate prenuclear palsy most commonly affects upgaze and downgaze together, but selective upgaze palsy or, more rarely, selective downgaze palsy may occur. Supranuclear vertical-gaze palsy is often due to ischemia in the most caudal territory of the thalamo–subthalamic artery and, less often, of the anterior choroidal artery, when this artery feeds the midbrain.[10] Vertical-gaze palsy may be due to midbrain hemorrhages, although this is far from common.[11,37,39,41] Combined upgaze and downgaze palsy often arises from upper-midbrain bilateral tegmental lesions, involving the riMLF, the INC, the PC or the periaqueductal gray matter due to either occlusion of midbrain–thalamic perforating arteries or because both paramedian arteries stem from one side or from a single pedicle, as occurs in a third of all brains.[15,16,42-45] A similar combined gaze deficit may occasionally result from unilateral lesions damaging the same areas as those produced by bilateral lesions.[15,17,33,46,47] Thus, unilateral lesions may affect the ipsilateral riMLF, as well as the contralateral riMLF fibers after their decussation, owing to damage of excitatory burst neurons of the riMLF.[17,33,46,47] Rapidity of all types of upward or downward eye movements may be affected or these movements may be lost altogether.[17,47] When a lesion is restricted to the riMLF, both vertical vestibulo–ocular reflex response and smooth-pursuit movements (SPMs) may be spared or impaired in only one direction.[15,16,45,48] Isolated upgaze palsy results from bilateral or unilateral lesions that involve the riMLF and the PC region and, thus, affect the INC or riMLF efferents traveling to the oculomotor neurons on both sides.[11,46,48,49] Unilateral infarcts may give rise to a functionally bilateral lesion of fibers at the PC level.[46] Small bilateral periaqueductal gray matter hemorrhages that spare the riMLF and the PC may produce this type of selective gaze palsy, although infrequently.[50] Although combined upgaze and downgaze palsy and isolated upgaze palsy have more frequently been reported with paramedian thalamic–subthalamic infarcts, purely midbrain infarcts may also produce these conjugate vertical-gaze palsies.[31] Selective downgaze palsy requires bilateral infarcts in the territory of the thalamo–subthalamic paramedian artery affecting the mediocaudal region of the riMLF,[10,16,46,51-53] although it may very rarely also be caused by small bilateral hemorrhages in the periaqueductal gray matter.[54] Other eye movement disorders [55-57]observed in midbrain or thalamo–mesencephalic infarcts are summarized in Box 1 . Disconjugate supranuclear eye movement disorders attributed to midbrain vascular lesions include monocular elevation palsy, crossed paralysis, the vertical one-and-a-half syndrome, skew deviation (SD), see-saw nystagmus (SSN) and V-pattern pseudobobbing.[10,11,46] Monocular elevation palsy (MEP; also known as double-elevator palsy) is a combined palsy of the elevator muscles reported in rostral midbrain infarcts. Ipsilateral MEP is due to interruption of efferents just after they leave the riMLF toward the oculomotor nuclear complex and before crossing. MEP can occasionally be accompanied by unilateral internuclear ophthalmoplegia (INO).[46,58] In contralateral MEP, ischemia affects riMLF fibers after decussating and before they reach the oculomotor nuclear complex.[46,59] Crossed vertical-gaze paresis is a very peculiar finding attributed to a unilateral mesodiencephalic junction infarct that impaired ipsilateral downgaze (monocular depressor paresis) and contralateral upgaze fibers (monocular elevator paresis) arising from the riMLF.[60] Moreover, a mild bilateral ptosis was found.[60] Nystagmus, which may also occur in midbrain stroke, mainly with lesions located at the mesodiencephalic junction, has a high localizing significance. Convergence-retraction nystagmus, associated with selective upgaze paresis and ipsilateral hypotropia, may, in rare cases, be the outstanding clinical finding as the result of a minute infarct strategically involving the periaqueductal region, the PC and the INC.[61,62] See-saw nystagmus is characterized by alternate elevation and depression of one eye accompanied by a similar movement in the other eye (but in the opposite direction). While one eye goes up and intorts, the other goes down and extorts. Although rare, jerk SSN (hemi-SSN) has also been reported with upper brainstem infarcts or hemorrhages, in which case the torsional component of the nystagmus fast phases rotates the upper poles of the eyes toward the side of the lesion.[63,64] A central disturbance of otholitic input has been identified as a pathogenic mechanism.[11,65] A dissociated vertical nystagmus (up- and down-beating) plus INO has been reported in a single patient with a tiny periaqueductal caudal midbrain infarct in the MLF region.[66] The vertical nystagmus was more prominent on the INO side.[66] Pendular SSN has been described in patients with unilateral mesodiencephalic infarcts or hemorrhages involving the INC.[17,65,67] Upbeat nystagmus may be observed after small midbrain ischemia located along the course of the ventral tegmental tract (Figure 2). [ CLOSE WINDOW ] Figure 2.39-year-old male patient with an upbeat nystagmus and a midline tegmental midbrain infarction. (A) Eye movements are normal in all directions of gaze. (B) Axial, sagittal MRI T2 sequences, sagittal T1 sequences and axial diffusion weighted images show a tegmental caudal midbrain infarction. In certain rare cases, a vertical impairment sparing a single direction in one eye may occur in bilateral or unilateral thalamo–mesencephalic infarctions.[68] The so-called vertical one-and-a-half syndrome is characterized by bilateral supranuclear impairment of all fast and slow downward eye movements on one side, combined with monocular paresis of elevation due to bilateral infarction.[68] The opposite may also occur: vertical upgaze palsy along with monocular depressor paresis on the side of the lesion,[46,69] or contralateral to the lesion,[46,69] has been described with thalamo–mesencephalic infarction, best explained by selective upward-gaze fibers at the PC level.[46] For downward-gaze palsy, a clear explanation has not been provided; nevertheless, an impairment before or after decussation in contralateral or ipsilateral lesions has been postulated.[46] Coexisting vertical and horizontal one-and-a-half syndromes have rarely been associated with ischemia involving the medial thalamus and the upper midbrain.[70] INO,[71-75] skew deviation[76] and ocular tilt reaction (OTR)[77,78] can be observed in midbrain infarcts, along with supranuclear gaze abnormalities Box 1 .[79] Nuclear or Fascicular Dysfunction of the Oculomotor & Trochlear Nerves in Midbrain StrokeOculomotor palsies are a common finding in middle midbrain infarcts; up to 90% of patients with ischemia affecting either the ventral or ventrolateral territories may have nuclear or fascicular third-nerve dysfunction.[31] These palsies result from occlusion of the small paramedian arteries of the BA when ischemia is the etiology, and may be the best indication of a midbrain stroke or be associated with other clinical signs of brainstem involvement.[10,31,36,80,81] Oculomotor nuclear complex lesions may have a constellation of clinical signs reflecting the particular topographical arrangement of the subnucleus and the selective vascular supply. They essentially present as complete homolateral oculomotor palsy together with contralateral superior rectus palsy (due to decussation of the fibers for superior rectus within the nuclear complex) and partial bilateral ptosis.[82,83] A nuclear lesion can have other forms of presentation.[11,84,85] Fascicular third-nerve lesions may have a form of presentation very similar to that found in peripheral etiologies; in both situations, signs of central neurological dysfunction are usually absent. Involvement may be partial or complete, depending on fascicular arrangement in the ventral midbrain.[31,86,87] Mostly, intra-axial lesions are attributed to infarcts or, much less commonly, to hemorrhages Box 1.[85-96] Contralesional hemiparesis, ataxia and involuntary movements along with ipsilesional intra-axial third nerve lesions constitute the different classic eponymous midbrain syndromes Box 1.[83,97-99] Nuclear or fascicular trochlear involvement is exceptional for two reasons: first, the dorsal position of the nucleus in the brain stem and, second, the fascicle's short intra-axial course. A superior oblique palsy contralateral to the lesion and a central Claude Bernard Horner syndrome on the side of the lesion may be characteristic and have a high localizing value.[11,100] Contralateral fourth-nerve palsy with a homolateral INO due to a small infarct in the caudal midbrain tegmentum has also been reported.[101] Eye Movement Disorders Occurring in Pontine StrokeAnatomical Basis of Eye Motor Control at the PonsMost brainstem networks participating in generation of saccadic and SPMs at the horizontal-axis level are located in the pontine tegmentum. The paramedian pontine reticular formation (PPRF), just ventral to the cranial nerve VI nuclei, is the prenuclear generator of ipsilateral horizontal saccades[19,102,103] and is currently also believed to play a role in saccadic movements in the vertical plane.[19,104] Neural circuits in this region also share in the generation of smooth-pursuit eye movements.[104] The excitatory premotor burst neurons (EBNs) and inhibitory premotor burst neurons (IBNs) reside at the PPRF level. The EBN, located rostrally to the abducens nucleus (AN), fire just prior to saccadic start and are considered to generate the saccadic pulse.[105-107] They send projections to the ipsilateral AN motoneurons and the internuclear neurons.[108] Inputs to EBN come from the contralateral superior colliculus and omnipause neurons in the nucleus raphe interpositus.[102,109] The IBNs are thought to suppress the activity of both EBNs and IBNs on the opposite side.[109] Outputs from both kinds of premotor burst neurons project to structures participating in the neural integrator's horizontal system: the nucleus prepositus hypoglossi (NPH) and the medial vestibular nucleus (MVN).[109] The omnipause neurons, located in the midline pons in the nucleus raphe interpositus, are also involved in the saccadic system.[110] They are tonically active, inhibiting both the EBNs and IBNs, and only cease their firing just prior to saccade onset, resulting in generation of the saccade in the contralateral medial rectus subnucleus of the third cranial nerve.[19,21,111,112] Saccadic signals to the abducens nucleus, therefore, derive from the ipsilateral PPRF.[112,113] The AN is considered the 'horizontal-gaze center', where saccadic, pursuit, vestibular and integrator commands converge.[114] It contains two intermingled groups of neurons, most of which correspond to the motoneurons innervating the ipsilateral lateral rectus muscle, while approximately a third of intermingled cells correspond to the internuclear neurons, with their axons decussating in the lower pons and running up through the MLF to synapse in the contralateral medial rectus subnucleus of the third cranial nerve (Figure 1).[18,19,115,116] Eccentric gaze-holding commands (also called the 'neural integrator') to the AN derive from the NPH and the MVN.[117] At the basis pontis, the dorsolateral pontine nucleus (DLPN) receives inputs from the temporal cortex and, in turn, projects to the contralateral flocculus. By contrast, the nucleus reticularis tegmenti pontis (NRTP) afferents originate in the frontal, supplementary, prefrontal and parietal eye fields. The NRTP sends signals to caudal fastigial nucleus and oculomotor vermis.[19,118-121] The MLF is a paired-midline brainstem white-matter fiber tract that travels the length of the midbrain and pons. It is a major pathway from the AN to the contralateral medial rectus oculomotor subnucleus.[122] The MLF is considered the final pathway for all classes of conjugate eye movements (Figure 1).[18] At the pontine level, the existence of three excitatory pathways participating in the upward vestibulo-ocular response, and operating in parallel and with complementary actions, has been proposed. The first pathway, the MLF, carries signals originated in the contralateral MVN and reaches the elevator muscle motoneurons of the nuclear oculomotor complex.[18] The second, the ventral tegmental tract (VTT), connects the superior vestibular nucleus (SVN) to the superior rectus and inferior oblique motoneurons on both sides of the oculomotor nuclear complex. At the lower pontine tegmentum, the VTT ascends in the posterolateral part and crosses the midline near the upper pole of the NRTP (at midpons level), then ascends in the anterior and medial part of the upper pontine tegmentum and lower midbrain tegmentum.[123-125] The third is the brachium conjunctivum, which is located very near the VTT at the lower tegmentum pontine. The participation of the brachium conjunctivum has been questioned and is probably more important in the transmission of vertical smooth-pursuit signals than the vertical vestibulo-ocular reflex.[123] Pontine Vascular SupplyVascular supply to the pons mainly comes from median and paramedian perforators (short and long arteries) issuing from the BA.[10,126] The AN, MLF and PPRF are irrigated by the long anteromedial arteries.[126] The anterolateral territory is fed by short circumferential arteries issuing from the BA.[126] The lateral territory is supplied from long circumferential arteries from the anterior inferior cerebellar artery (AICA) and the SCA at the lower and upper pons, respectively.[126] Pontine StrokeOne out of six posterior circulation ischemic strokes is confined to the pons.[126,127] Pontine hemorrhages are less frequent than infarcts, accounting for only 5–10% of all intracerebral hemorrhages.[128,129] Pontine infarcts are most often part of larger ischemias involving multiple territories in the vertebrobasilar system. Ventromedial infarcts (anteromedial and anterolateral territories) and lateral tegmental infarcts are the most common topographical patterns following restricted pontine ischemia.[126,130] Therefore, tegmental dysfunction and neuro-ophthalmological signs are very common, mainly when the caudal pons is involved. A broad spectrum of conjugate and disconjugate eye movement disorders occurs, often along with other clinical signs of tegmental damage. More rarely, eye movement disorders are isolated findings.[126,130] Eye Movement AbnormalitiesLesions located either in the AN or in the PPRF cause ipsilateral horizontal-gaze palsy (Figure 3).[126] This is a common finding, although it may be overlooked in ventromedial and lateral pontine infarcts.[126,131] Damage to the AN affect both motoneurons and interneurons; therefore, all saccadic movements of both eyes toward the side of the lesion are impaired, although vergence movements may be spared. Damage of the AN affects not only saccadic but also pursuit and vestibulo-ocular reflex (VOR). All conjugate eye movements toward the side of the lesion are impaired.[132,133] Nuclear VI involvement is often accompanied by either ipsilateral facial palsy or ipsilateral ataxia.[131,134,135] [ CLOSE WINDOW ] Figure 3.A 30-year-old male patient with bilateral horizontal-gaze palsy and an upbeat nystagmus. (A) Upward and downward gaze are normal, but no movement in either eye occurred when attempting to look horizontally to the right or to the left. (B) Brain computed tomography and axial and sagittal MRI T2 sequences show a large bilateral hematoma in the pons. Complete involvement of PPRF may result in loss of all fast conjugate movements to the ipsilateral side, often sparing SPM and VOR movements.[133] In instances of partial PPRF damage, saccadic movements to the ipsilateral side may be elicited; however, their speed is reduced.[136] Bilateral paramedian lesions of the PPRF cause bilateral horizontal saccadic paresis, but those in the vertical axis are preserved. There may be an associated ipsilateral sixth-nerve palsy.[136] On the other hand, a selective ipsilateral impairment of SPM may occur in tegmental pontine stroke.[137] The mechanism for this has been postulated to be infarcts or hemorrhages involving the suspected 'pursuit neurons' located in the vicinity of the AN.[137] Moreover, a selective impairment of SPM ipsilateral to the pontine ischemia may occur with lesions outside of the PPRF; in this case, a lesion affecting the DLPN or the NRTP.[124,138,139] Upbeat nystagmus has usually been reported in large and bilateral lesions involving the ventral tegmentum or the posterior basis pontis.[123] Nevertheless, a lacunar paramedian infarct at the upper pons level was reported to produce a primary position UBN as the main clinical finding.[124] Damage of the crossing ventral tegmental tract, just before and just after its decussation, could interrupt the excitatory upward vestibulo-ocular pathway, whereas the downward system would remain undamaged (Figure 3).[123] Pendular nystagmus (PN) can be associated with a palatal tremor, which is a segmental myoclonus characterized by a rhythmic involuntary jerking movement of the soft palate and the pharyngopalatine arch, often involving the diaphragm and laryngeal muscles.[140] In the setting of stroke, oculopalatal tremor (PN combined with palatal tremor) occurs several weeks or months after pontine stroke (infarct and hemorrhage), which may or may not be associated with cerebellar lesions.[140] PN can be symmetric or asymmetric in amplitude and have vertical, torsional or mixed trajectories. In the acute phase of stroke, other abnormal eye movements due to pontine or cerebellar lesion can usually be observed.[140,141] A disruption of the functional Guillain–Mollaret triangle (a dentate–rubro–olivary circuit) and its connections has been related to oculopalatal tremor.[140,142] Several mechanisms of oculopalatal tremor have been postulated, including damage of paramedian tract projections and subsequent denervation of the dorsal cap of the inferior olive, which may lead to instability of eye velocity-to-position integration, a loss of supranuclear inhibitory control followed by hypersynchronous firing of olivary neurons and impaired adaptation of the VOR due to degeneration of the inferior olivary nucleus, resulting in inappropriate oscillatory discharges by the floccular Purkinje cells.[140-144] Due to the close relationship of the AN with neighboring structures, lateral rectus palsy is rarely an isolated sign of pontine stroke. The responsible lesion is a minute infarct or hemorrhage exclusively affecting the AN fasciculus passing through the medial tegmentum to the basis pontis at the midpontine level rather than the AN.[93,145-148] Fascicular sixth-nerve palsy is most often associated with combined motor deficit, sensory disturbances, cerebellar signs or cranial nerve dysfunction. Nevertheless, the classic pontine crossed syndromes (Raymond–Cestan, Millard–Gubler or Foville syndromes) due to ischemia or small hemorrhages, and often associated with several neuro-ophthalmological findings, are infrequently observed Box 2.[149-152] Disconjugate disorders occurring in pontine stroke include INO, horizontal one-and-a-half syndrome, ocular bobbing (OB) and paralytic pontine esotropia. Internuclear ophthalmoplegia indicates MLF damage anywhere over its lengthy pontomesencephalic course between the AN and the contralateral oculomotor complex.[71] When stroke is the etiology, it mostly results from brainstem ischemia or, less often, from hemorrhaging.[71,153,154] Most infarcts causing INO occur at the pontomesencephalic junction or rostral pons level Box 2.[155-158] In approximately 10% of patients with infarcts restricted to the paramedian territory INO has been observed.[71] The essential clinical components of INO are limitation or slowing of adduction ipsilateral to the MLF lesion, often accompanied by dissociated contralateral horizontal jerk nystagmus in the abducting eye and preserved convergence.[153] A vascular etiology could be identified in approximately 40% of patients in an INO series.[153,154] In this setting, the initial complaint is often diplopia, which is most severe when the gaze is directed to the opposite side of impaired adduction.[71] Skew deviation, often with ipsilesional hypertropia, exotropia of the contralesional eye on the forward gaze, transient torsional-vertical nystagmus, jerky SSN and vertical-gaze limitation, may be concomitant neuro-ophthalmological findings in INO due to vascular etiology.[71,153,156,158,159] Unilateral INO most often occurs in pontine infarcts. Much less frequently, bilateral INO (rather more prevalent with multiple sclerosis) may occur with brainstem stroke.[71,154] There are often other associated brainstem symptoms and signs reflecting involvement of extrafascicular structures; nevertheless, unilateral or bilateral INO may occasionally occur in isolation.[17,72] Prognosis of INO related to stroke is variable. It may resolve, mainly when it is an isolated finding, or be long lasting.[159] Horizontal one-and-a-half syndrome is a peculiar sign considered specific for pontine tegmentum damage (Figure 4).[160-162] This syndrome is characterized by an ipsilesional horizontal-gaze palsy ('one') and INO ('and-a-half'), with abduction of the contralesional eye being the only preserved movement.[160] Oculocephalic reflexes on the opposite side may be preserved or absent, depending on the level of the damage. The oculocephalic response to the ipsilesional side (i.e., with head rotation to the contralesional side) may be preserved or absent, depending on whether the PPRF or abducens nucleus is lesioned, while the oculocephalic response to the contralesional side (i.e., with head rotation to the ipsilesional side) should be intact. In the upper ventrotegmental pons, oculocephalic reflexes are present, presumably because damage involves only the PPRF, whereas they are absent when the lesion is located at the caudal pons, owing to the involvement of both the sixth nucleus and the homolateral MLF.[160,163] Horizontal one-and-a-half syndrome is less common than INO in pontine ischemia.[126] A vascular etiology, mostly ischemia, has been demonstrated in approximately 50% of patients with horizontal one-and-a-half syndrome.[161] In some instances, the eye contralateral to the lesion assumes a position of abduction at rest, paralytic pontine exotropia.[162,164] It is usually associated with cranial nerve and long-tracts dysfunction. A variant of the horizontal one-and-a-half syndrome the 'eight-and-a-half' syndrome has been reported in pontine ischemia Box 2.[165] [ CLOSE WINDOW ] Figure 4.A 58-year-old male patient with arterial hypertension who exhibited a horizontal one-and-a-half syndrome due to a hematoma on the left pontine tegmentum. (A) Upward and downward gaze are normal, horizontal conjugate-gaze palsy to the left (the 'one'), adducting deficit in the left eye and abducting nystagmus in the right eye. (B) Brain CT and MRI scans show a hematoma on the left pontine tegmentum. An unusual form of abnormal vertical eye movement is OB. In its typical form, intermittent, spontaneous, usually conjugate downward jerk of both eyes may be observed, followed a few seconds later by a slow return to the mid-position. In this case, spontaneous or reflex horizontal-gaze movements are usually absent, whereas vertical-gaze movements are spared. The first cases reported (by Fisher in the 1960s) were vascular in etiology; two infarcts and one hemorrhage in the pons, pathologically verified.[166] Subsequently, OB (never in isolation) has, occasionally, been reported in both caudal pontine hemorrhage, extending in some instances to the medulla or midbrain and in large pontine ischemia.[167-169] In addition, OB has been reported in extrapontine hemorrhages or infarcts producing a compressive effect over the pons.[167,170] OB due to stroke has a variety of presentations (including typical and paretic or monocular) associated with concomitant contralateral ophthalmoplegia when the lesion extends to involve the third- or fourth-nerve nuclei or fascicles.[167,171] Indeed, the pathophysiological substrate is still controversial. Eye Movement Disorders Occurring in Cerebellar StrokeAnatomical Basis of Eye Motor Control at the CerebellumThe cerebellum plays an important role in eye movement control. Two separate parts of the cerebellum are thought to be involved in modulation of saccadic and SPMs. The first comprises the dorsal vermis and the underlying deep cerebellar nuclei (the dentate, fastigial and interpositus nuclei), and the second is the vestibulocerebellum (flocculus, paraflocculus, nodulus and ventral uvula). Thus, two parallel cortico–ponto–cerebellar networks, usually nonoverlapping, are devoted to controlling visually guided saccadic movements and SPMs.[120] For saccadic movements, the dorsal vermis and the caudal fastigial nucleus receive projections from the frontal eye field through the NRTP.[172-175] Then, the oculomotor vermis (the lobules VIc–VII) and their related Purkinje cells send projections toward the caudal part of the fastigial nucleus, which, in turn, outputs to contralateral brainstem gaze centers of the saccadic movements in the vertical and horizontal axes.[108,176–178] A similar cortico–ponto–cerebellar pathway operates for the smooth-pursuit system, but cortical areas subserving this system are smaller and differently located from their saccadic counterparts.[179] In addition, the lateral occipitotemporal cortex (the homologous medial temporal and the middle superior temporal areas in monkeys) also participates in smooth-pursuit control. This pathway is involved in pursuit maintenance. It projects to the DLPN and then to the contralateral dorsal vermis, flocculus and uvula. Another pathway, this one participating in pursuit initiation, descends from the frontal eye field and connects to the NRTP/pontine nuclei, terminating in the dorsal vermis, flocculus and uvula (Figure 1).[180] Finally, the flocculus takes part in VOR adaptive control and in the control of asymmetrical vertical slow phases (smooth-pursuit and vestibulo-ocular reflex movements).[142,181,182] Its efferents are directed to the vestibular nuclei, whereas afferents come from structures involved in the 'neural integrator'. The nodulus (mainly semicircular canal afferents) and the ventral uvula (mainly otolith afferents) are responsible for the 'velocity-storage' mechanism.[183] Vascular Supply of the CerebellumThe cerebellum is fed by three paired arteries according to its rostro–caudal topography. The SCA arises from the distal BA just inferior to the PCA bifurcation; its medial and lateral branches supply the superior vermis and the superior part of the cerebellar hemispheres, respectively. Deep branches irrigate the fastigial, intermediate and dentate nuclei, as well as most of the white matter.[184] The AICA originates from the lateral wall of the caudal third of the BA and feeds a small area of the anterior and medial cerebellum, the middle cerebellar peduncle and the flocculus; if the posterior inferior cerebellar artery (PICA) is hypoplastic, then the AICA take over the territory usually perfused by the lateral branch of the PICA.[184] Finally, the PICA most often issues from the distal intracranial vertebral artery (ICVA). The cerebellar branches of the PICA supply the inferior and posterior cerebellar hemisphere, the inferior vermis, tonsils, uvula and the nodulus.[184] Cerebellar StrokeCerebellar infarcts are far more frequent than hemorrhages.[185] Cerebellar ischemia accounts for approximately 2% of all brain infarcts and up to 30% of posterior cerebral infarcts.[186-190] Cerebellar hemorrhages range from 5 to 9% of all nontraumatic intracerebral bleeding.[191,192] Unilateral or bilateral territorial cerebellar infarcts do not occur in isolation, but most often coexist with other brainstem and supratentorial infarcts[190,193,194] or affect multiple cerebellar territories.[184,195,196] In the first case, the cerebellar clinical picture may be overshadowed by signs of brainstem or hemispheric dysfunction.[190] Although SCA infarcts are reported to be the most frequent,[195] it is likely that PICA and SCA infarct frequencies are almost equally distributed.[189,196-198] The clinical picture may be quite similar between the two main arterial cerebellar territories. In full PICA territory infarcts or PICA medial branch infarcts, signs that mimic a peripheral vestibular lesion and truncal ataxia predominate, whereas ipsilateral limb ataxia and lateropulsion, without dysarthria, are outstanding findings when ischemia involves the PICA lateral branch territory.[198-201] SCA territorial infarcts confined to the cerebellum are mainly characterized by dysarthria and limb and gait ataxia.[198,202] Eye Movement Disorders Due to Cerebellar StrokeNystagmus occurring along with other symptoms, is the most common oculomotor sign in cerebellar infarct, with frequencies between 15 and 75% reported.[189,196,197] Gaze-evoked nystagmus is a common finding in cerebellar infarcts and is usually associated with other ipsilateral cerebellar signs. The most frequent pattern is horizontal nystagmus in both gaze directions, with larger amplitude toward the side of the lesion. Nystagmus predominates slightly in patients with PICA over SCA territory ischemia Box 3 .[197] Nevertheless, when ischemia involves the PICA lateral branch, primary position nystagmus is often absent.[199] Downbeat nystagmus (DBN) is a clinically frequent finding characterized by an ocular motor disorder with a slow, spontaneous, upward eye drift and a compensatory fast phase directed downward.[203] DBN can be present in the primary position but, according to Alexander's law, is greatest when the patient looks down and can become more prominent in lateral gaze. It is often present with other oculomotor disorders (e.g., smooth-pursuit deficits, impairment of the optokinetic reflex and visual fixation suppression of the VOR). VOR deficits are also observed mainly in the vertical axis. The physiopathological mechanisms of DBN are still under debate, but it has been pointed out that asymmetry in the cerebello–brainstem network, which normally stabilizes vertical gaze, could lead to an imbalance in the vertical cerebello–vestibular 'neural integrator' and in vertical VOR tone, or to defective downward smooth pursuit.[123,142,204-210] Cerebellar infarcts in the PICA territory have been found to be the etiology in approximately 10% of patients with DBN.[203,207] Upbeat nystagmus is characterized by primary position nystagmus with the fast phase in an upward direction. It has rarely been reported in SCA infarcts.[202] Other forms of nystagmus with peculiar patterns have been reported, mainly in PICA territorial infarcts Box 3 . In periodic alternating nystagmus (PAN), the eyes exhibit primary position nystagmus, which, after 1–2 min, stops for a few seconds and then starts beating in the opposite direction.[211] The nodulus and the uvula maintain inhibitory control over vestibular rotational responses (velocity storage); therefore, a lesion at this level excessively prolongs the duration of the response by increasing the time constant of velocity storage.[142,211-213] PAN has most often been reported with midline cerebellar lesions[210] and infrequently in stroke damaging the cerebellum, either flocculonodular or nodular ischemia.[214-216] Rebound nystagmus is a horizontal primary position nystagmus that occurs when the eyes are returned to the primary position following sustained eccentric gaze. It has been associated with flocculus and paraflocculus lesions[217,218] or brainstem lesions damaging the MVN or the NPH.[219] It has rarely been reported, however, in cerebellar stroke.[219] A misperception of verticality (subjective visual vertical tilt) or, more rarely, of space (room-tilt illusion) has been reported, mainly with cortical or brainstem stroke, including bulbar lateral ischemia.[220] The exact mechanism linked to this unusual phenomenon is not fully understood, but a vestibulo–otolith–ocular dysfunction may be supported.[220] The flocculonodular system receives inputs from the labyrinth. The flocculus is usually irrigated by the AICA but, in less than 3% of cases, it is fed by the medial branch of the PICA.[188,221] A transient upside-down tilt illusion preceding the onset of symptoms mimicking a peripheral vestibular lesion may occur when the medial PICA supplies both the flocculus and the nodulus.[222] Box 3 summarizes other uncommon eye movement abnormalities observed in cerebellar stroke.[197,203,208,219,222,223] Eye movement Disorders in Medullary StrokeAnatomical Basis of Eye Motor Control at the MedullaThe medulla contains central vestibular pathways and structures mediating the gaze-holding mechanism. The complex neural network participating in the vestibular ocular motor function include the membranous labyrinth, which senses information about linear (the utricle and saccule) and angular (the semicircular canals) head acceleration.[224,225] Signals from the membranous labyrinth reach the homolateral vestibular nuclear complex at the pontomedullary junction. The SVN and the rostral part of the MVN receive semicircular canals information. The lateral vestibular nucleus receives projections from the utricle, and the ventral y-group vestibular nuclei receive projections from the saccule.[19] Secondary vestibular fibers from the SVN run into the ipsilateral MLF and reach the third and fourth cranial nerves, while similar projections arise from the MVN and travel into the contralateral MLF.[224,225] These pathways are responsible for the vertical VOR, and inputs to the vertical recti and oblique extraocular muscles are needed to produce vertical VOR.[224,225] From the MVN, efferents are directed bilaterally to the sixth-nerve nucleus and to the ipsilateral medial rectus subnucleus of the oculomotor nerve complex by way of the ascending tract of Deiters. These projections are responsible for the horizontal VOR.[225] In addition, outputs mainly from the inferior and medial vestibular nuclei are directed to the ipsilateral flocculonodular lobe, uvula and the fastigial nucleus by way of the juxtarestiform body.[225] The vestibulocerebellum and the vermis also have connections with the caudal and middle regions of the VN.[226,227] The VNs also have extensive reciprocal connections with the NPH and INC (the horizontal and vertical neural integrators, respectively), which are critical structures for gaze holding.[19,228] Medullary Vascular SupplyArterial supply to the medulla stems from the intracranial vertebral artery. Medial and paramedial branches from the proximal ICVA and the anterior spinal artery (ASA; a branch of the ICVA) supply the basal portion at the upper third and lower two-thirds medulla levels, respectively.[229,230] They irrigate several structures participating in eye movement control, including the MLF, NPH, paramedian tracts and the ascending efferent fibers from the VN.[229,230] The short circumferential branches of the ICVA feed most of the lateral tegmental portion, while the PICA, usually arising from the ICVA through perforating bulbar branches, irrigates the remaining lateral and posterior medulla en route to the inferior cerebellum, including the VN and the inferior cerebellar peduncle.[229,230] Medullary StrokeThe medulla is not affected by stroke as often as other brainstem structures.[230] Lateral medullary infarction (LMI; Wallenberg's syndrome) is the most common topographical pattern in medullary ischemia.[230-233] Owing to its dramatic presentation, it is also one of the best recognized among the brainstem stroke syndromes. Concurrent cerebellar or pontine infarcts may occur in LMI and sometimes constitute a challenge to defining the anatomical substrate of oculomotor disturbances.[10,234] A rostral extension of ischemia may occur in medial medullary infarcts (MMIs), allowing occurrence of oculomotor abnormalities due to pontine involvement.[235] Ischemia covering other arterial medullary territories (i.e., medial, bilateral lateral and hemimedullary) is more unusual.[233,236] The medulla oblongata is an exceedingly uncommon site of bleeding.[237] Eye Movement Abnormalities in Medullary StrokeA broad spectrum of oculomotor signs, sometimes in a complex pattern, may occur in medullary stroke, particularly in large and more dorsal LMI, and less often in bilateral medial infarcts.[10,238] Oculomotor signs may also be observed in MMIs when ischemia extends deep into the tegmentum dorsally. In hemimedullary infarct (Babinski Nageotte syndrome), the oculomotor findings resemble those found in LMI.[10,233] Subjective complaints in LMI may be vertical or oblique diplopia, fluctuating vertical diplopia, crossed diplopia (a form of double vision in which the false image is on the same side as the healthy eye), oscillopsia – mainly in the horizontal plane – and monocular or binocular blurred vision.[10,238] Ipsilateral Horner syndrome is the most common pupil/eyelid abnormality in medullary infarcts; up to 90% of patients with a LMI and 15% with a MMI have ipsilateral Horner syndrome, due to involvement of the descending sympathetic fibers coursing through the lateral reticular substance.[232,239-241] Nystagmus is common in medullary stroke and generates distinct patterns. It may be observed in two-thirds of patients with LMI, mainly when ischemia covers the lower medulla.[237,244,245] Damage to the caudal VN, the vestibulocerebellar connections and the pathways running in the inferior cerebellar peduncle have been the suggested pathomechanisms.[234,239,240] In LMI, nystagmus in the primary position usually beats away from the lesion side or, less often, toward the ipsilateral side of the lesion, and is often more pronounced in the dark.[234,242-244] It may be horizontal or torsional.[238,239,242,245] Ipsilesional nystagmus occurs when the superior vestibular nucleus or the rostral region of the MVN are damaged, while it is contralesional if the vestibular nerve root and caudal parts of the VN are impaired.[246] Spontaneous nystagmus occurring in Walllenberg syndrome may result from otolith and semicircular canal imbalance.[244,247] In large LMIs, mainly, horizontal-torsional (or horizontal-torsional vertical) nystagmus may also occur occasionally.[10,244] In torsional nystagmus, the upper pole of the iris beats away from the lesion.[245] Contrary to what usually occurs in LMI, spontaneous nystagmus mostly beats to the side affected by ischemia in MMI.[231] Gaze-evoked horizontal nystagmus, which is most prominent when looking to the side opposite the lesion has been reported in upper medullary tegmentum infarcts[236,248] and in LMI.[233,240,249] Upbeat nystagmus, either in primary position or more marked on upward gaze, has been described with unilateral or bilateral MMI, usually when the lesion extends to the dorsal region affecting the MLF or the perihypoglossal nuclei (consisting of the nucleus prepositus hypoglossi, nucleus of Roller and the nucleus intercalatus of Staderini).[230,250-253] Upbeat nystagmus in primary position or gaze evoked, nevertheless, has seldom been reported in LMI.[234] Other uncommon forms of nystagmus have also been reported in medullary stroke Box 4 .[231,249,254-258] Other less-known oculomotor signs resulting from impairment of otolith vestibular connections may occur in LMI, some of which are only demonstrated after symptoms and signs due to semicircular canal dysfunction subside.[255] These signs include SD (a vertical ocular misalignment of prenuclear origin), ipsilesional OTR, tilt of subjective visual verticality, environmental tilt and positional nystagmus. SD characterized by ipsilateral hypotropia is a common finding in patients with Wallenberg syndrome.[238,242,244,259,260] Moreover, partial or complete OTR (a triad of SD, head tilt and ocular torsion) may also be observed in LMI.[239,244,260,261] In OTR, excyclodeviation of the hypotropic eye with little or no incyclodeviation of the contralateral hypertropic eye may be observed.[245,261] Asymmetrical damage at the level of the VN of vertical VOR pathways ending in the ocular motor subnuclei in the midbrain is thought to be the mechanism.[255,262] Tilt of subjective visual verticality with an ipsilesional displacement of 9–9.8° and gradual disappearance of this phenomenon after several weeks of ischemia has been observed in LMI.[232,238,255,263] Less often, a sensation of environmental tilt with 90–180° inversion of the visual images (floor-on-ceiling phenomenon), attributed to a disturbance of vestibular otolith connections, may also be observed in LMI.[233,243] Ocular lateropulsion of Barré, a tonic conjugate eye drift toward the side of the ischemia, may be found in approximately a half of patients with LMI and is usually accompanied by axial lateropulsion.[10,255,264,265] In this case, the reflex and voluntary movements are full. This oculostatic manifestation is inhibited by fixation; therefore, it may more often be observed when the eyes have been previously closed and, more rarely, with the patient's eyes open.[10,255,264,265] Moreover, the ocular lateropulsion may mimic a conjugate eye deviation from a hemispheric lesion. Ocular ipsipulsion in LMIs has been attributed to climbing fiber damage from the contralesional inferior olivary nucleus to the dorsal vermis.[255,264-266] Oculodynamic manifestations may also accompany ocular lateropulsion, sometimes as an isolated finding. Ipsilateral horizontal saccades become hypermetric, while those to the contralateral side are hypometric.[10,232,255] Vertical saccades are also affected and may show an ipsilesional torsipulsion.[243,265,267] Impaired SPM away from the side of ischemia may occur, while those toward the side of lesion are usually normal. An infarcted inferior cerebellar peduncle in Wallenberg's syndrome results in inhibition of the ipsilateral fastigial nucleus, producing saccadic ipsipulsion.[267] Expert CommentaryNumerous brain regions, all the way from the cortex down to the brainstem and the cerebellum, are involved in the generation and control of eye movements. Over the last few decades, significant progress has been made toward understanding the anatomical, physiological and chemical substrates of the oculomotor system, which creates a final output signal to execute the different classes of human eye movements. To date, the premotor component of the oculomotor system is probably one of the best understood. One important advance has been the conceptual evolution in the model of the neural networks subserving saccadic and pursuit eye movements. Traditionally, these networks were viewed as anatomically distinct systems, but recent research has demonstrated that they share many common neural elements in their brainstem circuitry and at higher cortical and subcortical levels. The study of vertebrobasilar stroke has improved knowledge of the pathophysiological basis of eye movement disorders. In this line, some eye movement disorders considered to be either idiopathic, or not produced by stroke, have recently been attributed to small lesions of vascular origin, most often infarcts, disclosed by modern neuroimaging techniques, such as high-resolution MRI and functional neuroimaging. In the clinical setting, these small lesions have provided the opportunity to propose clinical pathophysiological models regarding the different neural networks in the brainstem and cerebellum, as well as connectivity with other levels participating in eye movement control. These clinical models, in turn, have served to develop computational models to be tested in experimental settings to increase knowledge of this intricate system. Interaction between experimental models and stroke patients with eye movement abnormalities has led to a better understanding of how the brain controls eye movements. Important advances have occurred in the understanding of the neural pathways of saccadic, pursuit and vestibulo-ocular reflex movements. A better understanding of the physiopathological aspects of nystagmus has also been achieved, particularly with concern to upbeat and downbeat nystagmus and pendular nystagmus associated with palatal tremor. Furthermore, posterior circulation stroke is not uncommon. Lesion localization in vertebrobasilar stroke can be a daunting task, mainly when small stroke lesions fail to produce long-tract signs or typical signs of posterior circulation involvement. The complex vascular architecture of the vertebrobasilar system makes the brainstem, in particular, susceptible to small lesions that cause a broad spectrum of neuro-ophthalmological signs. In clinical settings, eye-movement abnormalities may have specific anatomical correlates that can act as clues for accurate localization at different levels of the brainstem and cerebellum. In daily clinical practice, eye movement disorders caused by stroke can, in most cases, be identified at the bedside, although they often go unrecognized. Five-year ViewDespite growing understanding of the organization of oculomotor control, many critical questions remain to be answered. For example, the brainstem vestibulo–oculomotor pathways are not yet fully known, the clinical implications of lesions involving the reticular formation have not been completely settled and therapeutic approach in patients with disabling or long-lasting eye movement disorders remain to be explored. Therefore, future developments in this area will, hopefully, answer these critical issues in the experimental and clinical contexts. To date, increasing application of functional imaging techniques, such as PET scans, functional MRI and MRI tractography, has provided in vivo information about neuronal pathways. In stroke-induced eye movement disorders, these techniques will produce further information for better comprehension of the effect of stroke lesions on the circuitry serving the eye-movement system, since they make it possible to establish direct clinical–radiological correlations. Based on the trend observed in both community- and hospital-based stroke registries over the last 25 years, the number of patients suffering vertebrobasilar stroke will continue to grow in the coming years. Although modern imaging techniques that are best suited for early visualization of stroke (including small infarcts and hemorrhages) in the brainstem and cerebellum, will be more available, knowledge, timely recognition and appropriate clinical interpretation of the broad variety of eye movement disorders associated with vertebrobasilar stroke will be no less important. |
 |
| DOWNLOAD OPHTHALMOLOGY TOOLBAR |
الثلاثاء، يونيو ١٦، ٢٠٠٩
Oculomotor Disorders in Vertebrobasilar Stroke
الاشتراك في:
تعليقات الرسالة (Atom)



ليست هناك تعليقات:
إرسال تعليق